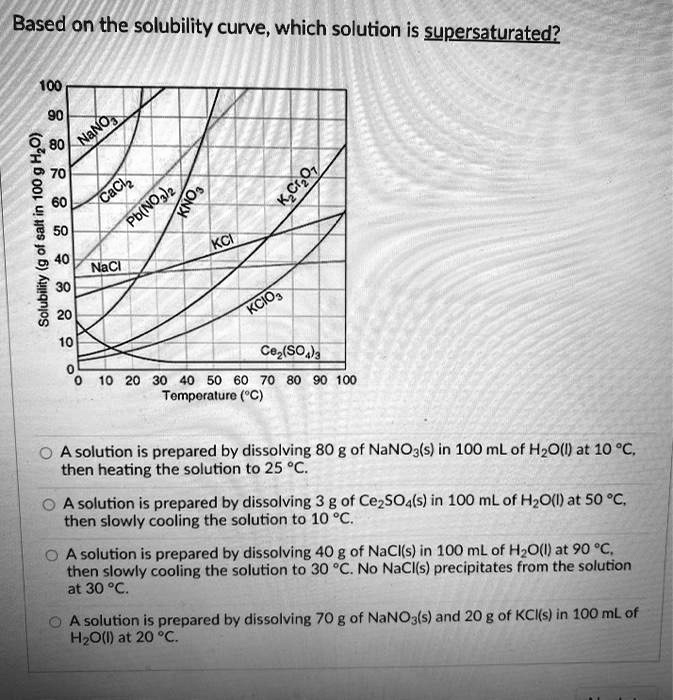
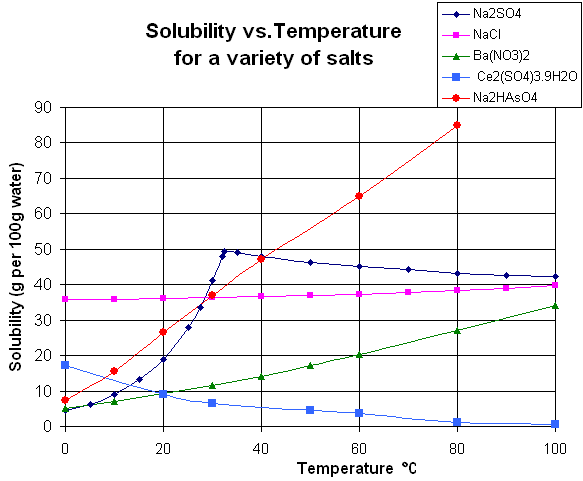
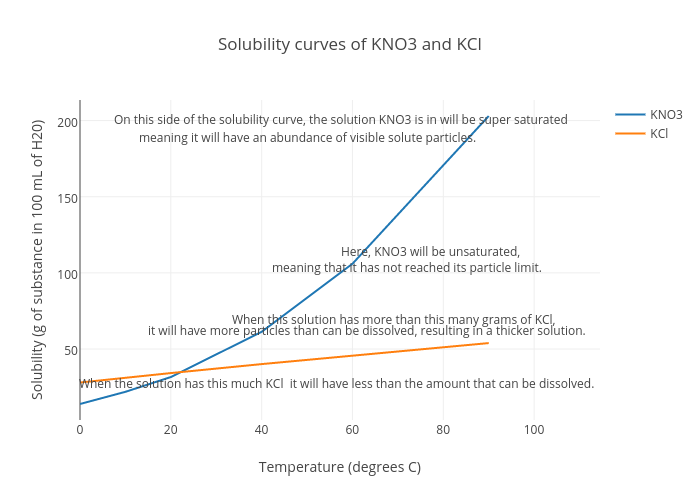
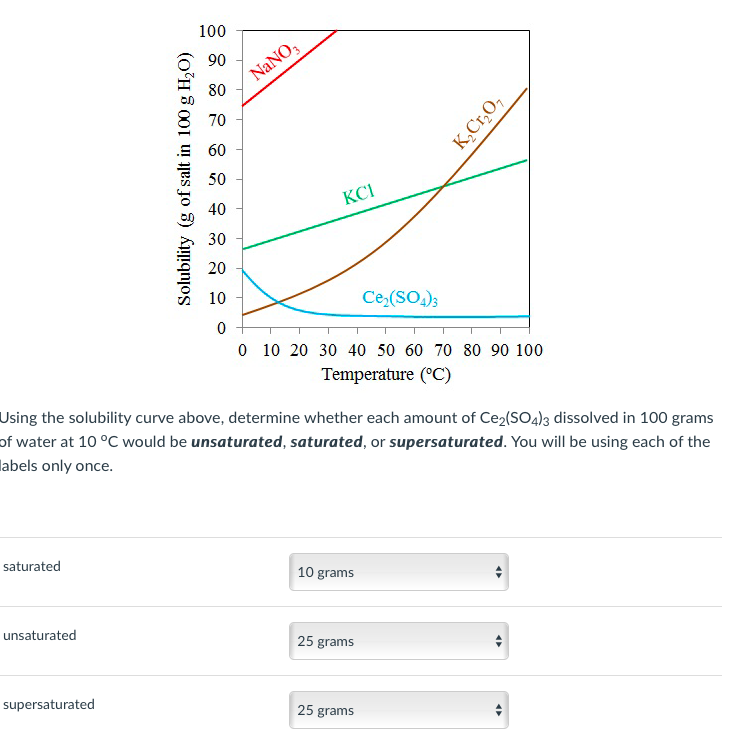
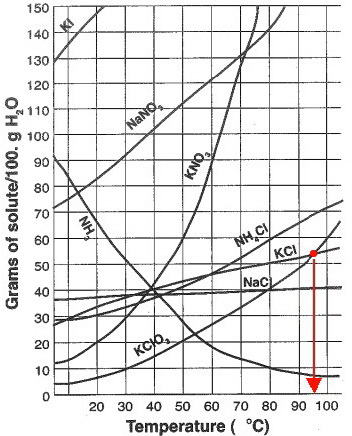
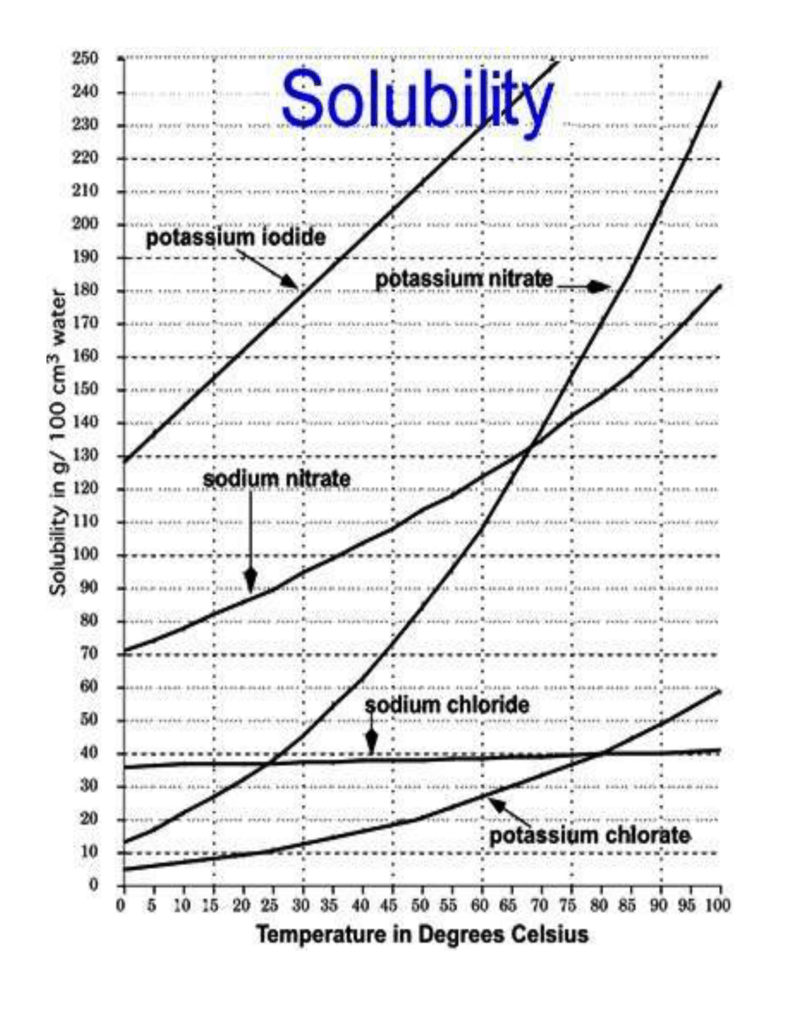
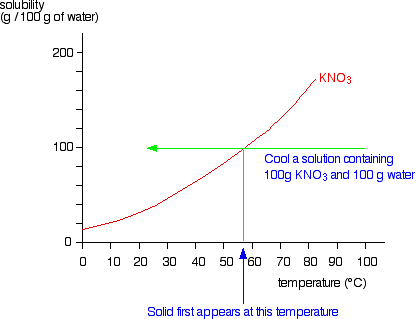
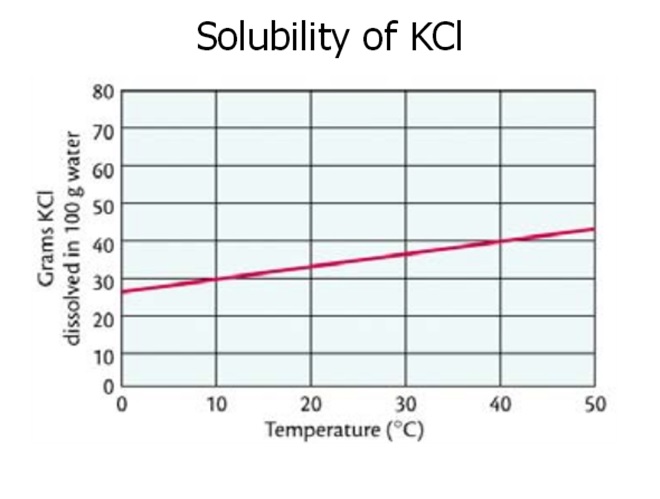
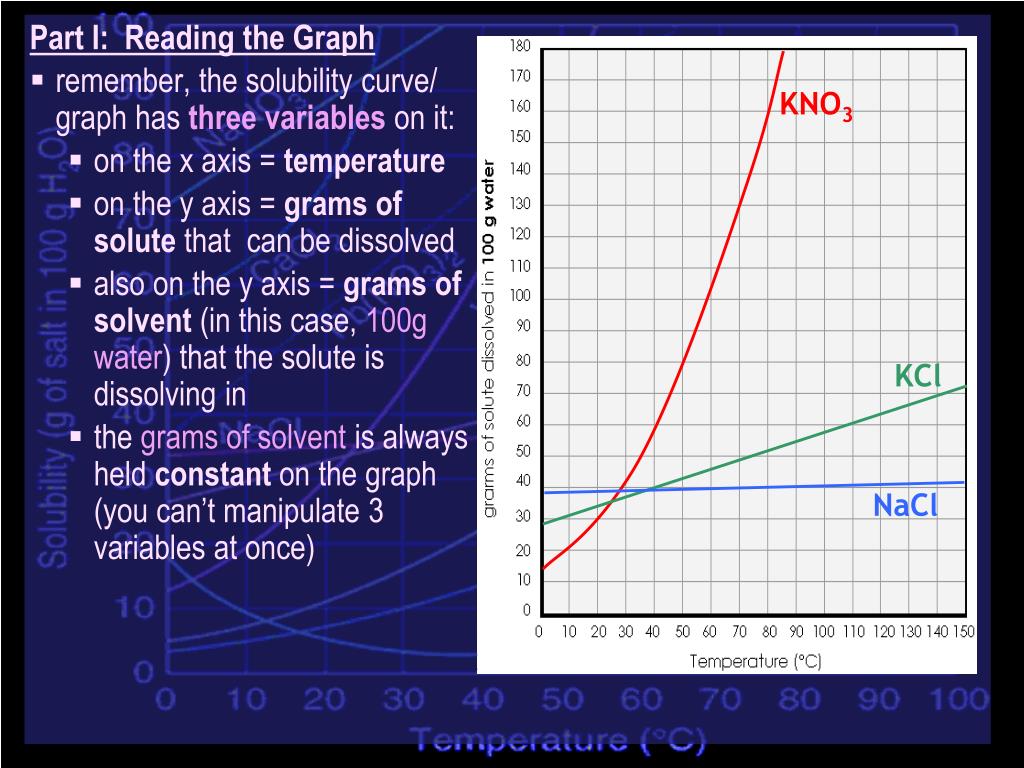
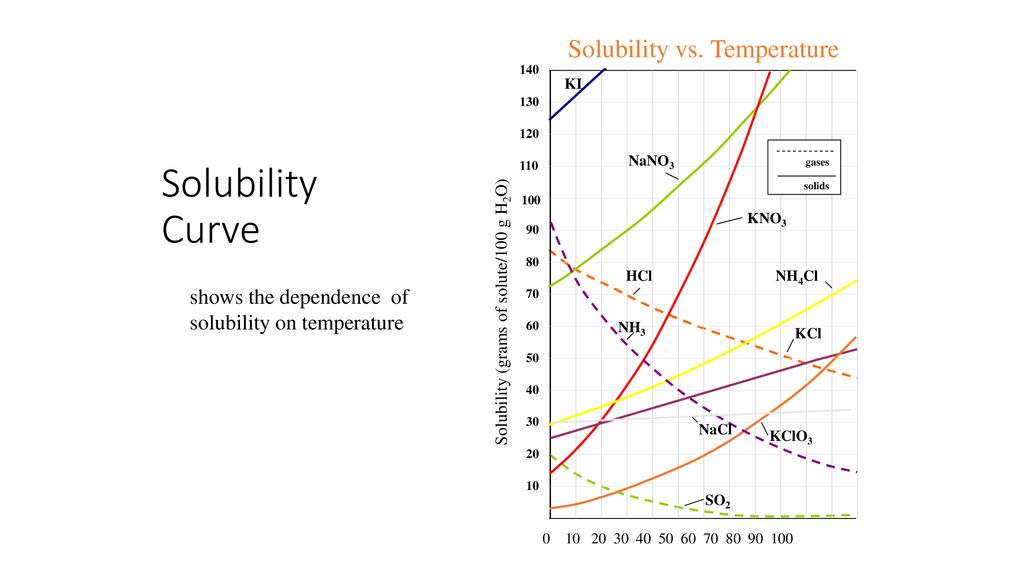
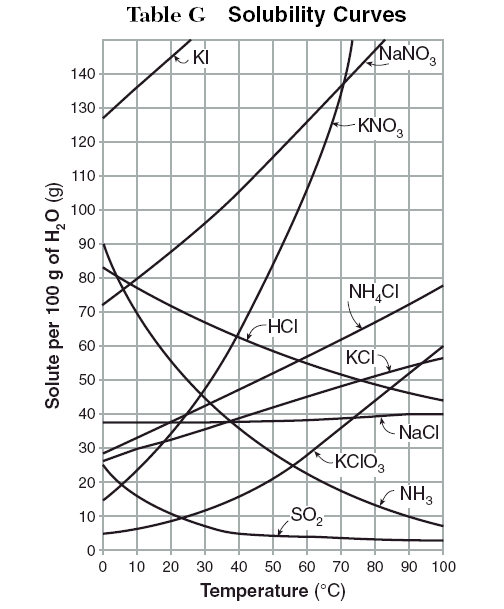
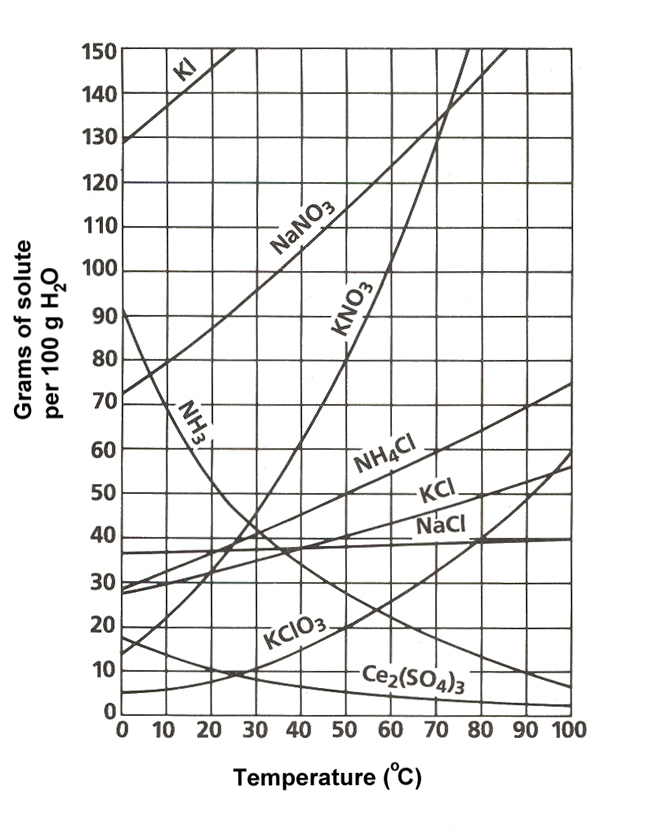
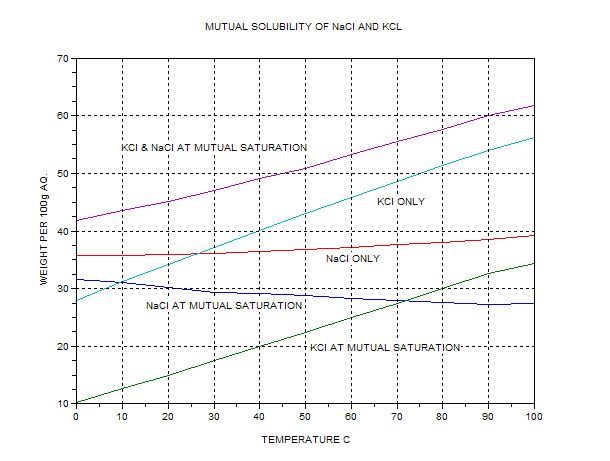
At which temperature do solutions chloride and potassium chloride have the same solubility? | Homework.Study.com
Data Collection: Experiment 4a – Measure the Solubility of Potassium Chloride Experiment 4b – Measure the Solubility of Ammo
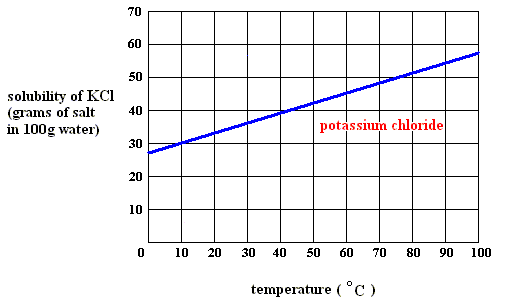
At what temperature is the concentration of a saturated solution of KCl (molar mass 74.5 g) approximately 3 molal? | Socratic

Figure 1 from Growth and characterization of bis ( thiourea ) potassium chloride crystals for NLO applications | Semantic Scholar

Solubility and solvation thermodynamics of dl-nor-valine in aqueous solutions of NaCl and KCl - ScienceDirect